Safety in adults

CRYSVITA clinical safety profile in adults with XLH
Adverse reactions (>5%) in CRYSVITA-treated patients and in ≥2 patients more than with placebo in the 24-week placebo-controlled period of Study 41
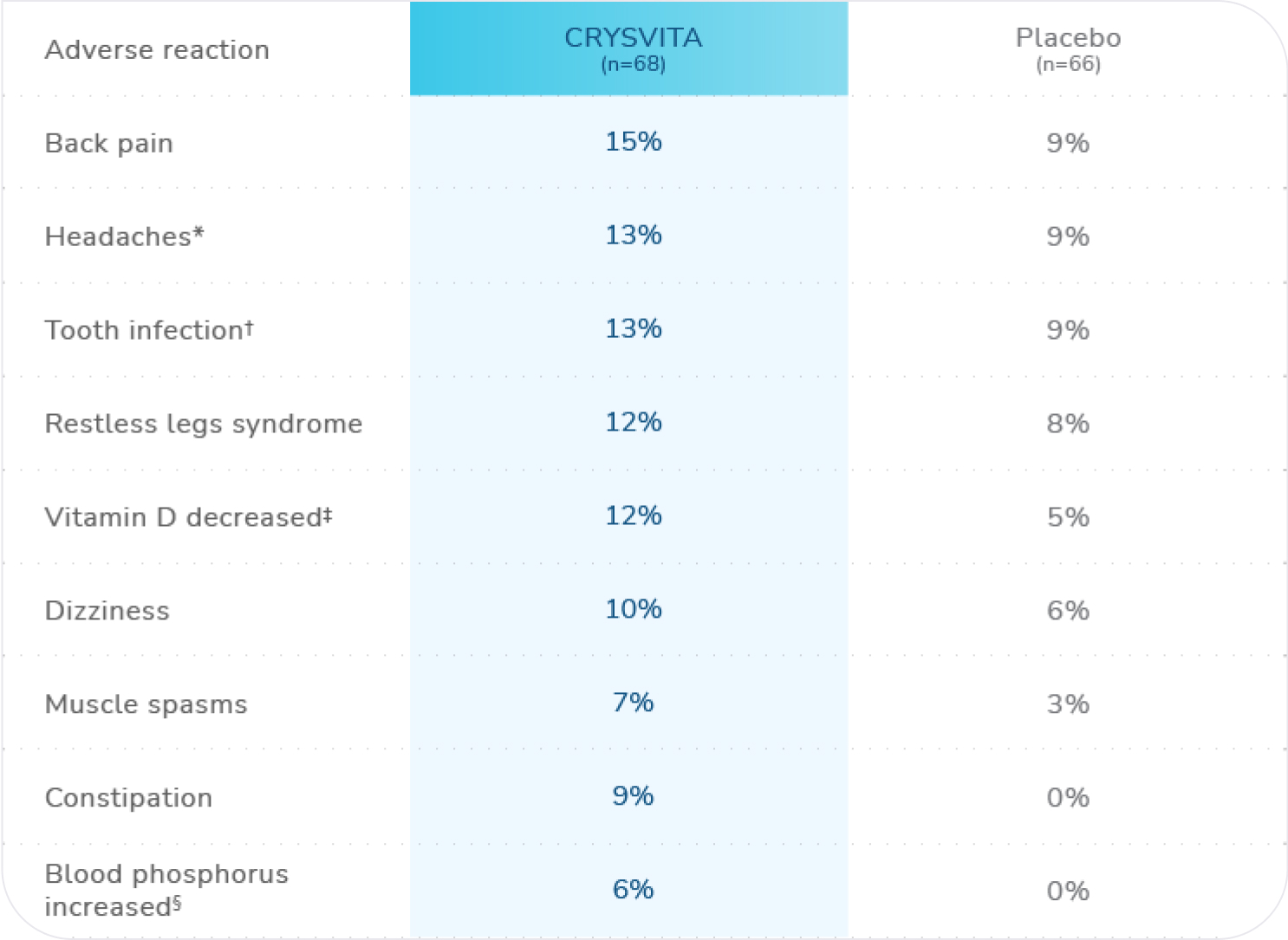
n=total number of patients who received at least 1 dose of CRYSVITA or placebo.
*Headache includes: headache and head discomfort.1
†Tooth infection includes: tooth abscess and tooth infection.1
‡Vitamin D decreased includes: vitamin D deficiency, blood 25-hydroxycholecalciferol decreased, and vitamin D decreased.1
§Blood phosphorus increased includes: blood phosphorus increased and hyperphosphatemia.1
The 24-week placebo-controlled study was followed by a 24-week open-label treatment period in which all patients received CRYSVITA subcutaneously every 4 weeks. No new adverse reactions were identified in the open-label extension period.1

Hypersensitivity reactions
In the double-blind period of Study 4, approximately 6% of patients in both the CRYSVITA and placebo treatment groups experienced a hypersensitivity event. The events were mild or moderate and did not require discontinuation.1

Hyperphosphatemia
In the double-blind period of Study 4, 7% of patients in the CRYSVITA treatment group experienced hyperphosphatemia, meeting the protocol-specified criteria for dose reduction (either a single serum phosphorus greater than 5.0 mg/dL or serum phosphorus greater than 4.5 mg/dL [the upper limit of normal] on 2 occasions). The hyperphosphatemia was managed with dose reduction. The dose for all patients meeting the protocol-specified criteria was reduced by 50%. One patient required a second dose reduction for continued hyperphosphatemia.1

Injection site reaction
In the double-blind period of Study 4, approximately 12% of patients in both the CRYSVITA and placebo treatment groups had a local reaction (eg, injection site reaction, erythema, rash, bruising, pain, pruritus, and hematoma) at the site of the injection. Injection site reactions were generally mild in severity, occurred within 1 day of injection, lasted approximately 1 to 3 days, required no treatment, and resolved in almost all instances.1

Restless legs syndrome (RLS)
In the double-blind period of Study 4, approximately 12% of the CRYSVITA treatment group had worsening of baseline RLS or new onset RLS of mild to moderate severity; these events did not lead to dose discontinuation. Nonserious RLS has also been reported in other repeat-dose adult XLH studies; in 1 case, worsening baseline RLS led to drug discontinuation and subsequent resolution of the event.1

Spinal stenosis
Spinal stenosis is prevalent in adults with XLH, and spinal cord compression has been reported. In the CRYSVITA phase 2 and phase 3 studies of adults with XLH (N=176), a total of 7 patients underwent spinal surgery. Most of these cases appeared to involve progression of a preexisting spinal stenosis. It is unknown if CRYSVITA therapy exacerbates spinal stenosis or spinal cord compression.1
Ready to start your patients on CRYSVITA?
Take the first step by filling out the enrollment form.

Stay connected
Set up time with a representative to talk more about CRYSVITA,
or sign up for more information on CRYSVITA for the treatment of XLH.
