The Radiographic Global Impression of Change (RGI-C)
RGI-C is a 7-point scoring method (-3=severe worsening; 0=no change; +3=near/complete healing) designed for comprehensive evaluation of skeletal health.1,2
- RGI-C scores assess changes in the severity of rickets using the disease-specific qualitative RGI-C scoring system7
- RGI-C scores are assigned by 3 independent experts blinded to dose and patient2
- An RGI-C score of +2.0 indicates substantial healing of rickets1
RGI-C scoring scale2




The Thacher Rickets Severity Score (RSS)
RSS is a 10-point score for radiographs of wrists and knees to assess the degree of metaphyseal fraying and cupping and the proportion of the growth plate affected.1,2,8
-
It is a predefined scale that evaluates specific abnormalities in the wrists and knees1,2,8
- Total points progress in half-point increments from 0-10: wrists (0-4) plus knees (0-6)
- RSS is assigned by an independent expert blinded to dose and patient1,2,8
- Higher scores indicate a more severe state of rickets, and a reduction in RSS indicates an improvement in severity1,2,8
An example of RSS scores from knee X-rays8
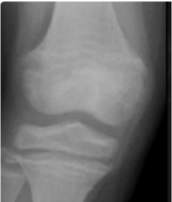
RSS=1.0
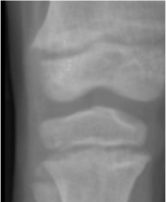
RSS=1.5
The standing height z-score1,3
- The standing height z-score was used as a measurement for growth
- The “stature-for-age” z-score was determined based on a percentile basis using the Centers for Disease Control and Prevention/National Center for Health Statistics (CDC/NCHS) Clinical Growth Charts
- Standing height was used to calculate the stature-for-age z-score based on standardized age- and sex-adjusted stature from the CDC
Efficacy in children
Actor portrayal

A phase 3 study showed that CRYSVITA1:
- Helped heal rickets and reduce rickets severity
- Increased growth
- Increased and sustained serum phosphorus levels
Study design
CRYSVITA was studied in a 64-week randomized, open-label phase 3 study (Study 1) in 61 children with XLH between 1 and 12 years of age. Study 1 compared treatment with CRYSVITA (n=29) every 2 weeks to conventional therapy (n=32) that included oral phosphate and active vitamin D supplements. Patients randomized to CRYSVITA received a mean dose of approximately 0.90 mg/kg (range 0.8-1.2 mg/kg) every 2 weeks.1
Primary endpoint:
- Healing of rickets at week 40
- As assessed by Radiographic Global Impression of Change (RGI-C) scores
Secondary endpoints:
Change from baseline, comparing CRYSVITA with conventional therapy in:
- Lower extremity skeletal abnormalities
- As assessed by the RGI-C long leg score
- Severity of rickets
- As measured by total Thacher Rickets Severity Score (RSS)
- Growth
- As measured by standing height z-score
- Serum phosphorus levels
- Alkaline phosphatase (ALP) activity
Safety: Number of subjects with adverse events (AEs), serious adverse events (SAEs), and AEs leading to discontinuation.
CRYSVITA was evaluated in 2 other phase 2 studies. To learn more about Study 2 and Study 3, please see full Prescribing Information.
Disease burden at baseline1
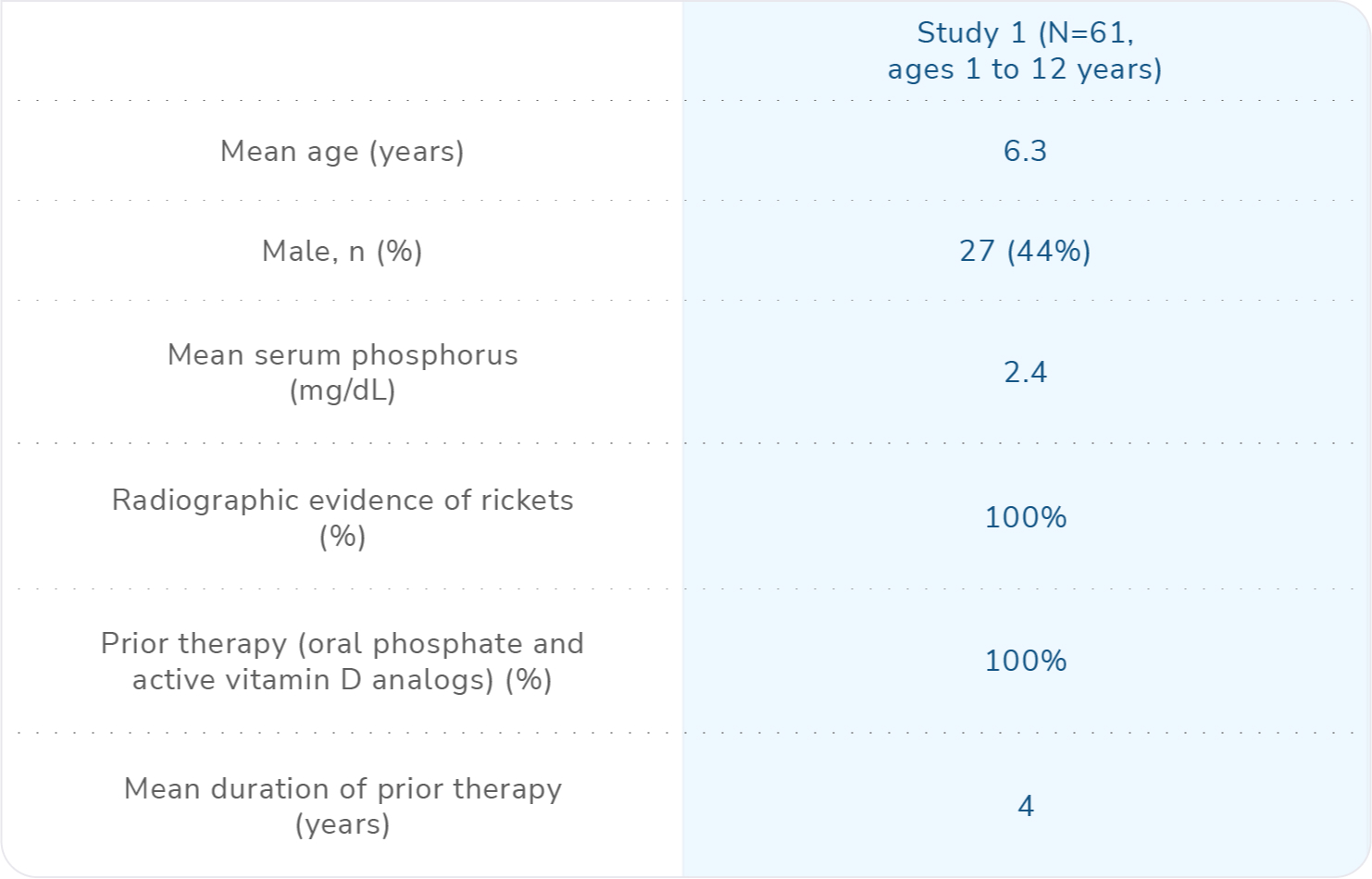
No pediatric patients discontinued CRYSVITA treatment in the study.1
RICKETS HEALING
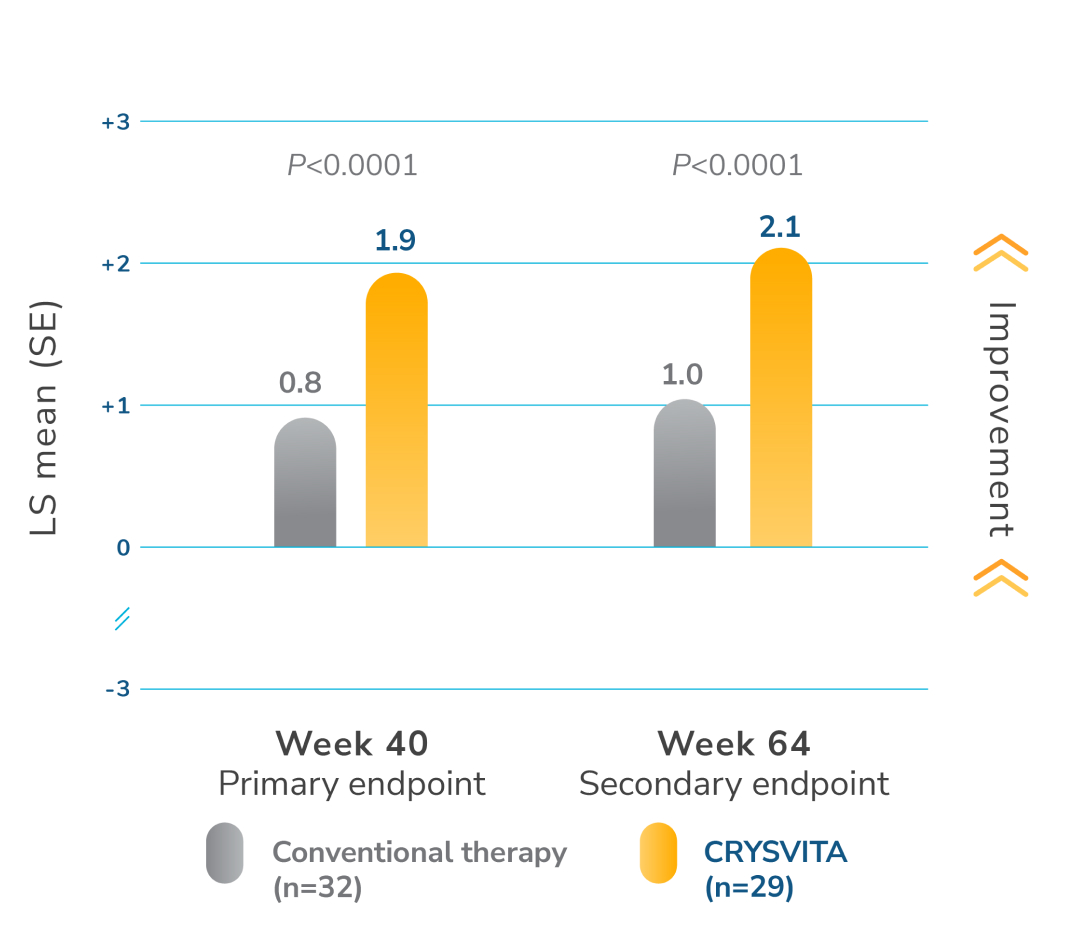
CRYSVITA significantly improved healing of rickets compared with conventional therapy1,2
*RGI-C at week 40 was the primary endpoint of the study. The estimates of LS mean for week 40 are from an ANCOVA model accounting for treatment group, baseline RSS, and baseline age stratification factor. The estimates for week 64 are from a GEE model accounting for treatment group, visit, treatment by visit interaction, baseline RSS, and baseline age stratification factor.1
ANCOVA=analysis of covariance; CI=confidence interval; GEE=generalized estimation equation; LS=least squares; RGI-C=Radiographic Global Impression of Change; RSS=Thacher Rickets Severity Score; SE=standard error.
At week 40, mean RGI-C score in patients treated with CRYSVITA was +1.9, indicating that healing of rickets occurred. Twenty-one out of 29 patients achieved an RGI-C score of ≥+2.0, indicating substantial healing of rickets. At week 64, patients treated with CRYSVITA maintained a mean RGI-C global score of +2.06.1,2
RICKETS HEALING
CRYSVITA helped significantly more patients achieve substantial healing of rickets compared with conventional therapy1,2

of patients receiving CRYSVITA achieved substantial healing† of rickets

of patients receiving conventional therapy
†An RGI-C score of +2.0 indicates a substantial healing of rickets.1,2
At week 40, 72% (21 out of 29) of patients achieved substantial healing (RGI-C score of ≥+2.0) of rickets compared with 6% (2 out of 32) of patients receiving conventional therapy (P<0.0001). These results were maintained at week 64.1,2
RICKETS HEALING
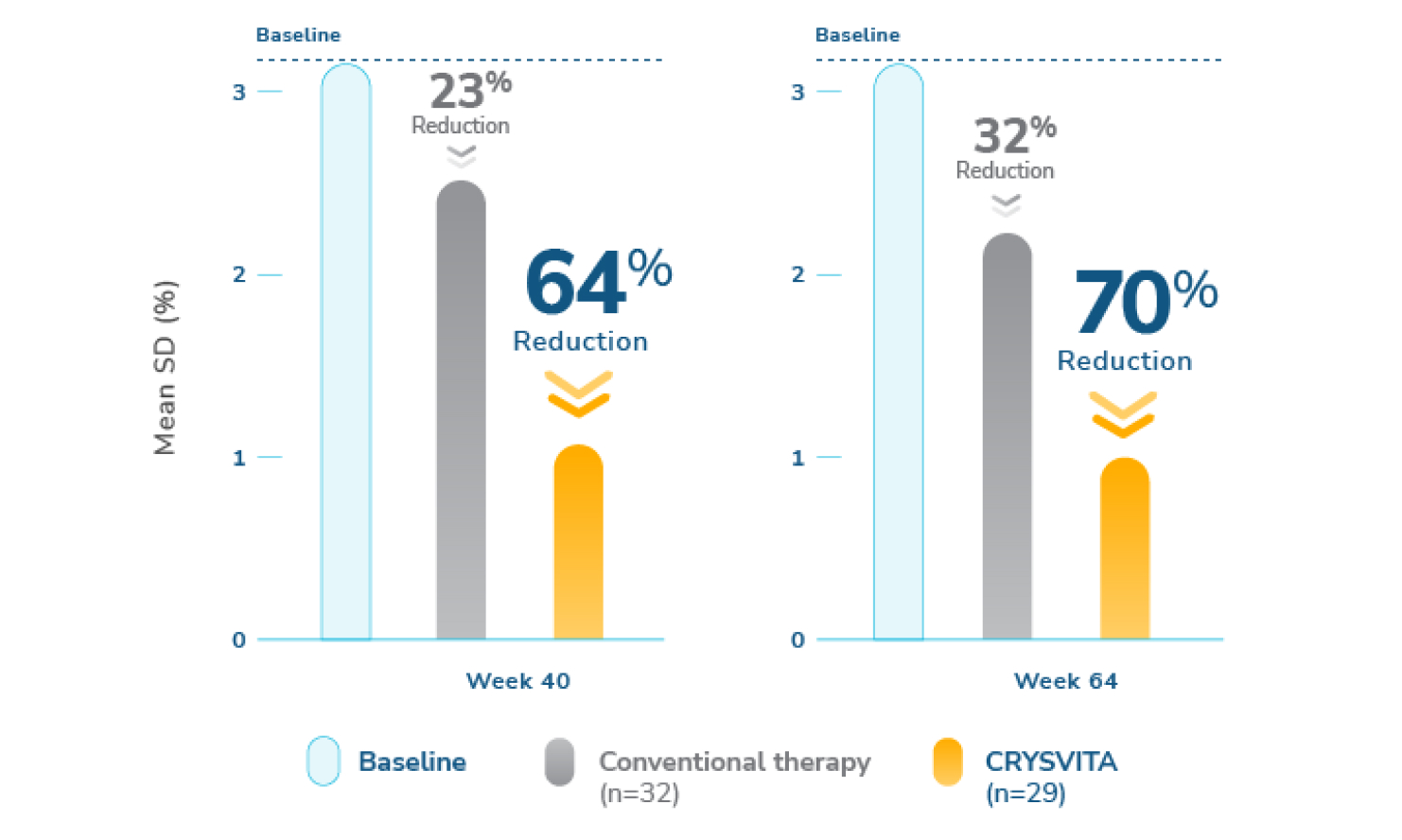
CRYSVITA led to a 64% reduction in rickets severity from baseline to week 40 and maintained at week 641-3
‡ The estimates of LS mean for week 40 are from an ANCOVA model accounting for treatment group, baseline RSS, and baseline age stratification factor. The estimates for week 64 are from a GEE model accounting for treatment group, visit, treatment-by-visit interaction, baseline RSS, and baseline age stratification factor.1,2
Treatment every 2 weeks with CRYSVITA showed superior reduction in mean total rickets severity, compared with conventional therapy, as assessed using the Thacher Rickets Severity Score (RSS). A reduced RSS score indicates improvement in rickets severity.1,2
CRYSVITA demonstrated greater improvement in lower extremity skeletal abnormalities1,2
At week 64, CRYSVITA maintained greater improvement in lower extremity skeletal abnormalities compared with conventional therapy (LS mean [standard error]: +1.25 [0.17] vs +0.29 [0.12]).1,2§
§ The estimates for week 64 are from a generalized estimating equation (GEE) model accounting for treatment group, visit, treatment-by-visit interaction, and baseline age stratification factor.1-3
Radiographic examples of lower extremity skeletal abnormality improvement with CRYSVITA
The radiographic examples are of a patient with XLH (female, 2.9 years of age) who received CRYSVITA every 2 weeks for 64 weeks, compared with a patient (also female, 2.9 years of age) who continued on conventional therapy.1,4
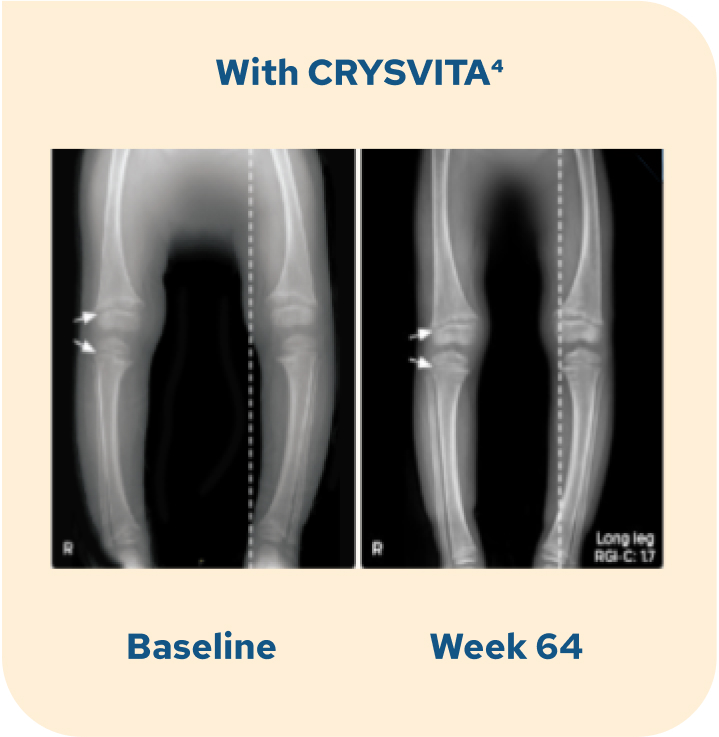
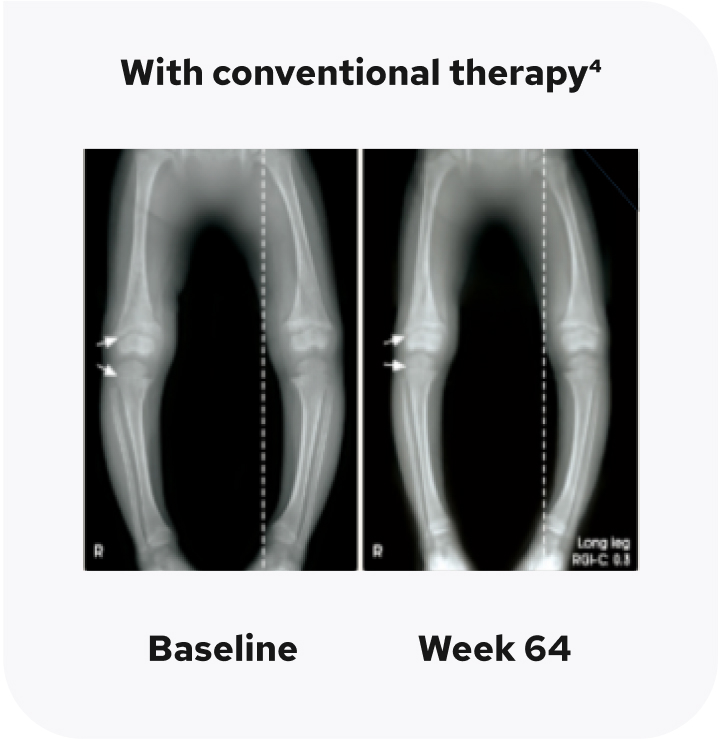
Individual results may vary.
GROWTH INCREASE
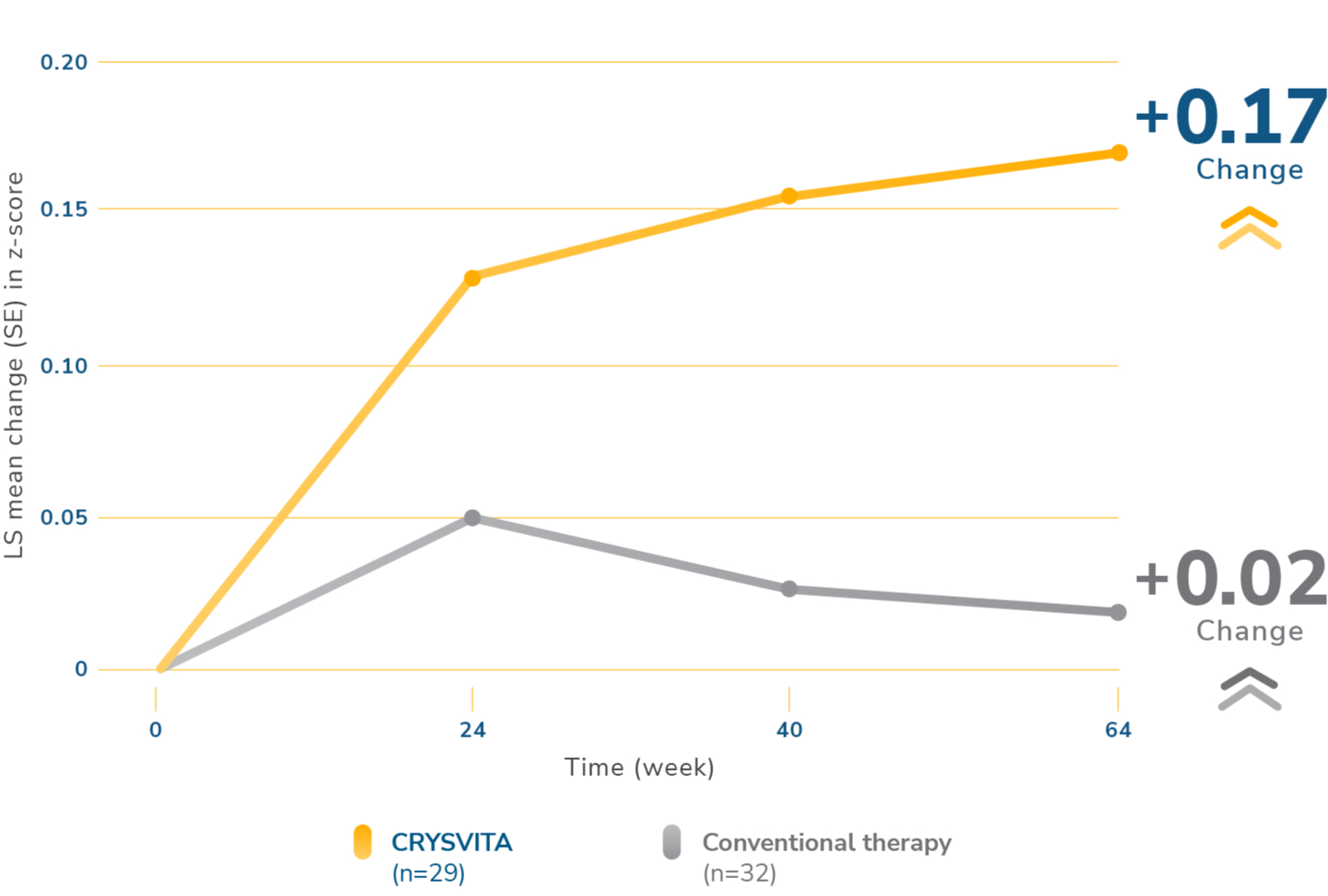
CRYSVITA significantly increased growth compared to conventional therapy1,2
||The estimates of LS mean, SE, and 95% CI are from a GEE model, which included change from baseline for recumbent length/standing height z-score as the dependent variable, treatment group, visit, interaction between treatment group by visit, and baseline RSS stratification as factors; and age and baseline recumbent length/standing height z-score as continuous covariates, with exchangeable covariance structure.2
CI=confidence interval.
At 64 weeks, CRYSVITA treatment increased standing mean (SD) height z-score from -2.32 (1.17) at baseline to -2.11 (1.11) in patients who received CRYSVITA every 2 weeks compared with a mean (SD) height z-score increase from -2.05 (0.87) at baseline to -2.03 (0.83) in patients who received conventional therapy.1
CHANGE IN SERUM PHOSPHORUS LEVELS
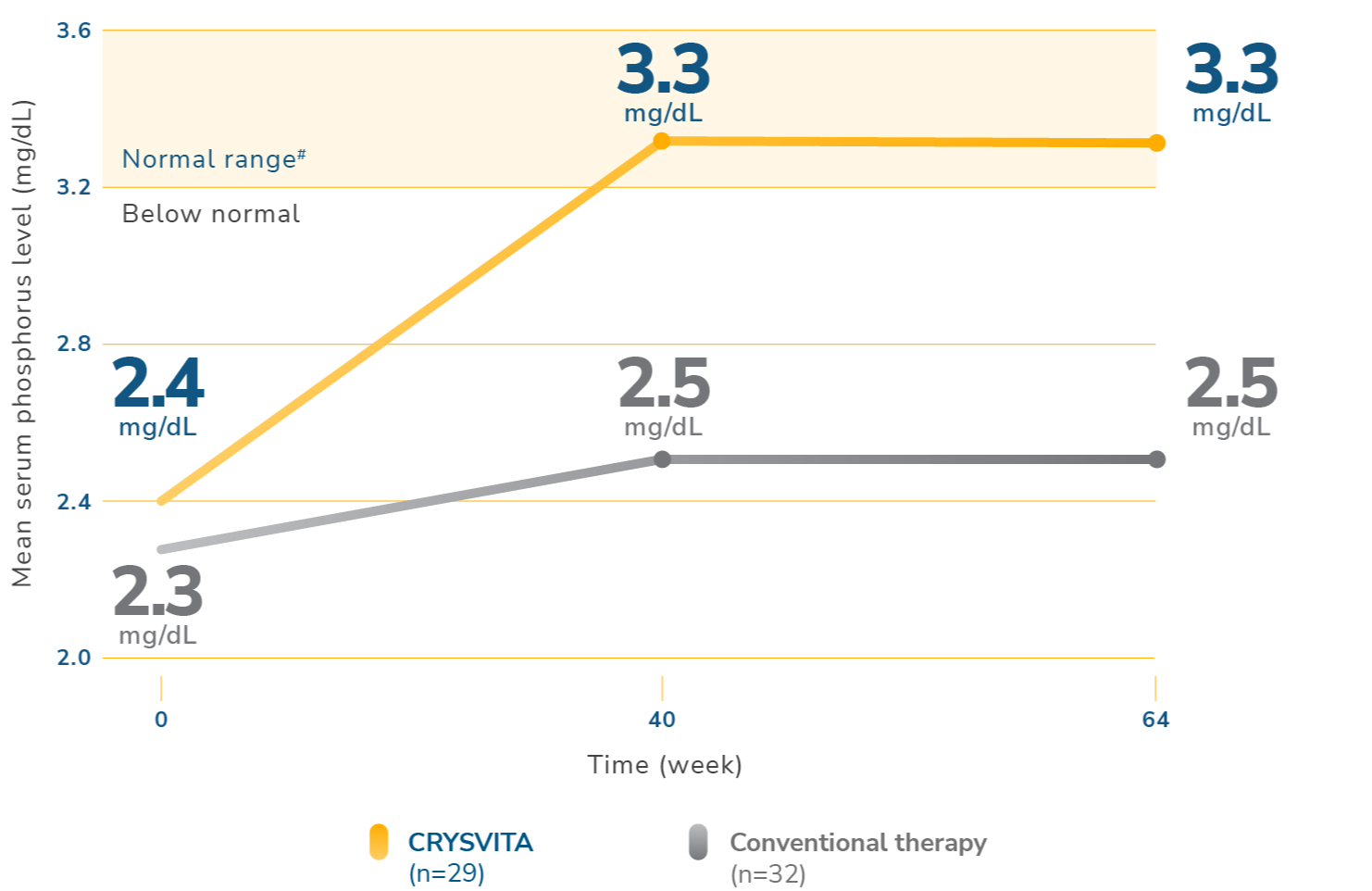
CRYSVITA increased and maintained serum phosphorus levels1,2
¶Serum phosphorus level (mg/dL) (mean ±SD). Lower limit of normal (LLN) is 3.2 mg/dL.1
#Normal levels of serum phosphorus range from 3.2 mg/dL to 6.1 mg/dL. Note that the normal levels of serum phosphorus vary by age and sex.3,5,6
SD=standard deviation.
An increase in serum phosphorus levels compared with conventional therapy was observed with CRYSVITA at week 2 and maintained at week 64.1,2
CRYSVITA increased and sustained the tubular maximum for phosphorus reabsorption per glomerular filtration rate (TmP/GFR) compared with conventional therapy from baseline to week 641
Ready to start your patients on CRYSVITA?
Take the first step by filling out the enrollment form.

Stay connected
Set up time with a representative to talk more about CRYSVITA,
or sign up for more information on CRYSVITA for the treatment of XLH.